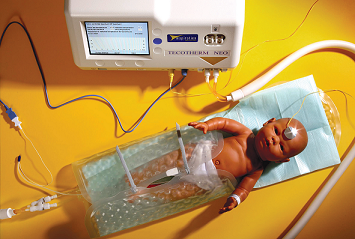
Our division participated in a multicenter study testing a cooling blanket on newborns with hypoxic ischemic encephalopathy (HIE), a major cause of death and severe disability. The blanket is now being used on these patients.
Research is an integral part of the Division of Neonatology at Rady Children’s Hospital and UC San Diego, as it provides the opportunity to find better therapies and treatment strategies to improve patient care. The goal of our research is to improve the quality of care and outcomes for babies.
Throughout our history, division investigators have made important discoveries related to surfactant, inhaled nitric oxide and neonatal resuscitation. Our current team is researching the benefits of human milk, protecting brain development, preventing infection and using genomic data for precision medicine.
During your baby’s stay here, a member of the research team may approach you about current available studies. Although studies may not provide any direct benefits to your baby, we believe the success of these studies will be beneficial for future patients. Participation in research is entirely voluntary. Whether or not you decide to have your baby participate in one or more of the available studies, it is our honor and privilege to continue to provide your baby with the best possible care. Be sure that all of your questions have been answered and that you feel comfortable and informed prior to making any decisions.
Questions to Ask
About the Study
- What is the purpose of the study?
- What kinds of therapies, procedures and/or tests will my baby have during the study?
- How is the study different from regular care?
- How are study results and safety of babies being checked?
- How long will my baby be in the study?
- Can my baby be in more than one study?
Possible Risk and Benefits
- What are the possible short-term and long-term benefits for my baby?
- What are the risks if I choose to have my baby participate?
- Are there any risks if I choose not to have my baby participate?
- What happens if I change my mind about having my baby participate in the study after I signed the consent form?
Costs
- Will I have to pay for tests or study drugs?
- Will I be paid for my baby’s participation in the study?
The division is active in many different types of research. Our research projects are vetted by physicians and the patient care team. All studies are regulated by our Human Rights Protection Office (HRPP) to ensure patient safety and to facilitate high quality and ethical research. If you have questions about your baby’s rights in a study, you can contact the HRPP by email (hrpp@ucsd.edu) or by phone (858-246-4777).
For more information on our research, visit the UC San Diego Department of Pediatrics website.